
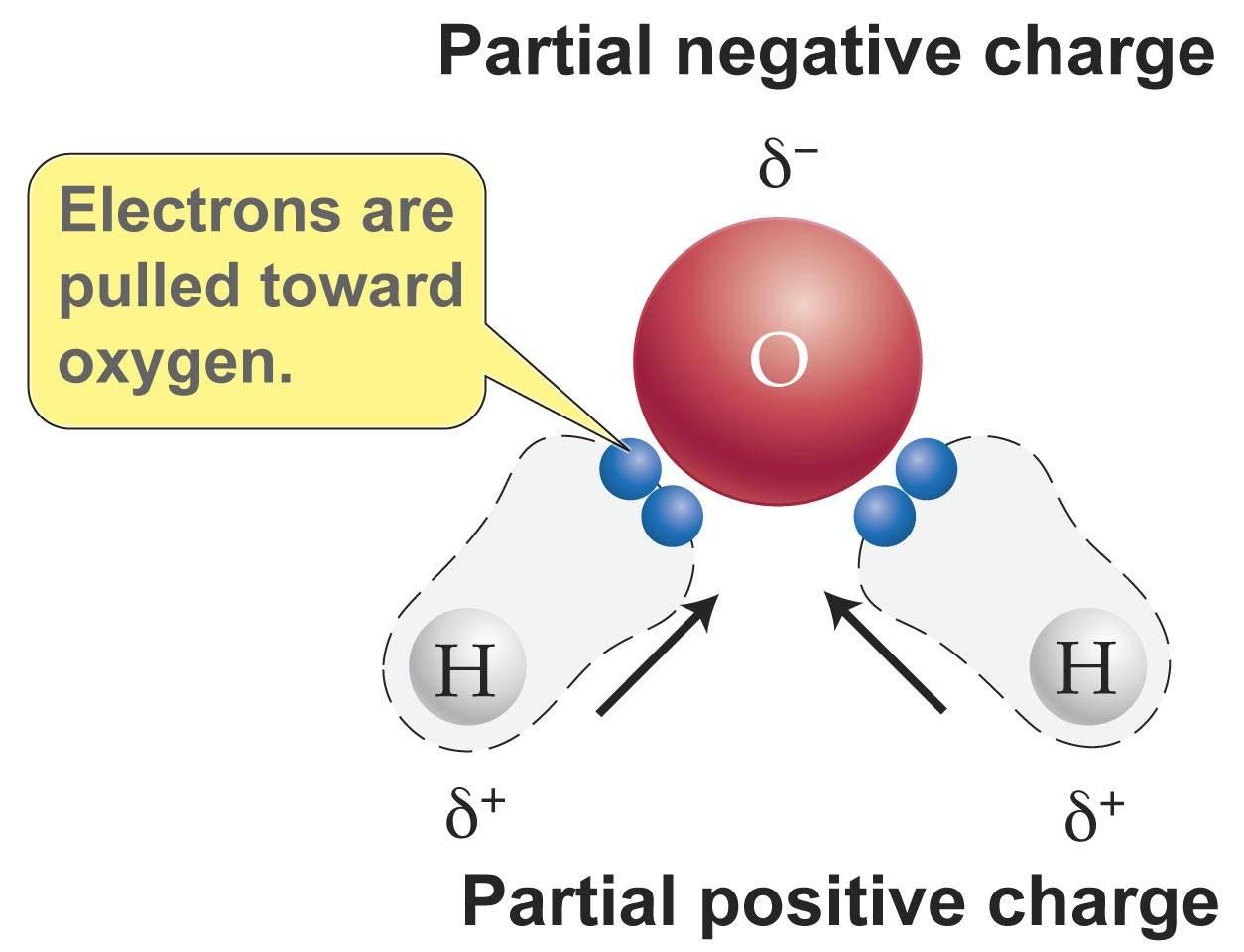
In a liquid, the molecules have enough kinetic energy to keep moving around. To understand what causes ice to float but solid wax to sink, let’s think first about what happens when a liquid turns to a solid (again, the States of Matter module can be a handy review here). (To review density and buoyancy, see our Density module) This isn’t a common state of affairs if you put a chunk of solid wax into a vat of molten wax, it will sink toward the bottom (and possibly melt before it gets there). Its ability to bob to the top of the water line means that the ice (water in its solid state) is less dense than liquid water. If you have some ice cubes, drop one in your glass. Now it’s time to make use of that glass of water. Properties of water that arise from hydrogen bonding It floats! As the illustration shows, each water molecule forms attractions with four other water molecules, a network of connections that makes the hydrogen bonding in water particularly strong and lends the substance its many unique properties. If you look at the central molecule in this figure you see that the oxygen end of the molecule forms hydrogen bonds with two other water molecules in addition, each hydrogen on the central molecule is attracted to a separate water molecule. There are many other compounds that form hydrogen bonds, but the ones between water molecules are particularly strong. Yet, they have a big effect on how water behaves. In fact, they are often referred to as an attractive force as opposed to a true bond. These bonds are relatively weak compared to other types of covalent or ionic bonds. Hydrogen bonds make water molecules "stick" together. The slight negative charge on the oxygen atom is attracted to the slight positive charge on a hydrogen atom. As a result, a partial negative charge (ð-) forms at the oxygen end of the molecule, and a partial positive charge (ð+) forms at each of the hydrogen atom ends (Figure 1).įigure 2: Hydrogen bonds between water molecules. Our Chemical Bonding: The Nature of the Chemical Bond module discussed how a dipole forms across a water molecule in the bond between oxygen and hydrogen, the electrons are shared unequally, drawn a bit more to the oxygen. Those interactions are, in turn, related to how the atoms within a water molecule interact with each other. The amount of energy required to go from solid to liquid and liquid to gas is related to how water molecules interact with each other. As you might recall (or can read about in our module on States of Matter), water molecules are in a different energy state in each phase. For starters, water is the only substance that exists naturally on our planet as a solid (ice and snow), liquid (rivers, lakes, and oceans), and a gas (water in the atmosphere as humidity).
But it can’t exist without water.ĭespite its scarcity across the universe, water is so abundant on Earth that we aren’t always aware of how special it is. Life, it seems, can tough it out in acid, lye, extreme salt, extreme heat, and other conditions that would kill us humans.

Water is at once so vital and so scarce that exobiologists (scientists looking for life beyond Earth) set their sights on planets where water might exist. Water is so central to our existence that you might be surprised to learn that it’s a rare and unusual substance in the universe. Water is absolutely essential to our lives on Earth. Water puts out fires, cooks our food, makes our soap get sudsy, and hundreds of other things. You use water to wash yourself, your clothes, and your car. For one thing, your body can’t function more than a few days without it. Got your glass? Now take a sip and think about all the roles water plays in your life. By the time you’ve reached the end, you’ll have a much greater appreciation for this miracle liquid. For the previous version, go here.īefore we start, get yourself a glass of water. This is an updated version of the Water module.


 0 kommentar(er)
0 kommentar(er)
